Westmed Inc
Neo-Pod T Operators Manual Rev 03
Operators Manual
20 Pages
Preview
Page 1
Neo-Pod™ “T” Heat & Humidification Transport System Operators Manual
The Neo-Pod “T” manual is translated into the following languages and can be found on our website at: www.westmedinc.com French German Italian Portuguese Spanish
ENGLISH TABLE OF CONTEXT
Page
Description Indications Contraindications Components Neo-Pod “T” Controller Power and Controller Cable Assembly Quick Connect Cable Airway Temperature Sensor Setup Procedure Cable Assembly Attachments Breathing Circuit Connections Starting and Stopping Treatment Controls Alarms Display Cleaning and Maintenance Servicing Specifications Warranty Gross Dimensions of Controller Classification according to IEC 60601-1 Tables General Warnings and Disposal
2 2 2 2 3 3-4 4 4 5 5 6 7 7 8 8 9 9-10 10 10 10 11 11-14 15
1
Description: The Neo-Pod “T” Transport Humidification System and associated breathing circuit is a wick-type respiratory gas-heated humidifier system intended for infant use during transport in an appropriate transport incubator. The system is designed to heat and humidify dry medical gas. Indications: The Neo-Pod “T” with LavaBedTM Humidifier Cartridge and associated breathing circuits are indicated to deliver heated and humidified gas during infant transport. The Neo-Pod “T” Transport Humidification System is for use only as recommended by a physician. Contraindications: There are no contraindications to providing physiologic conditioning of inspired gas during patient transport with High Flow Nasal Cannula Therapy. Components: The Neo-Pod “T” Transport Humidification System consists of the Neo-Pod “T” Controller, LavaBed Humidifier Cartridge, Power and Controller Cable with Airway Temperature Sensor, Pole Mount Bracket and Mattress Bracket.
2
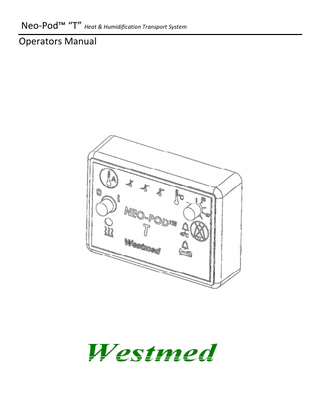
1. Neo-Pod “T” Controller: (Figure A) The Neo-Pod “T” Controller is the electronic unit that controls the LavaBed Humidifier Cartridge and monitors the temperature of the system.
Caution! Read all instructions prior to use. Caution! Ensure the correct cable assembly is selected for your specific application. Cable assemblies are sold separately.
2. Power and Controller Cable Assembly: The Neo-Pod “T” is powered by a 12 volt DC power source and can utilize two types of cable assemblies. These include the DC power cables and the AC power converter cable as shown on page 4.
3
Option 1. For use with the Transport Incubator 12 VDC Internal Battery Supply. See Instructions for Use included with cable assembly.
PN 9400
Option 2 For use with a 120~240 VAC to 12 VDC Power Cable. See Instructions for Use included with cable assembly.
PN 9401
• The Quick Connect Cable provides power to the LavaBed Humidifier Cartridge. • The Airway Temperature Sensor is an optional connection that monitors patient airway temperature when the Airway Temperature button is depressed and held. 3. Additional Components • Pole Mount Bracket • Mattress Bracket
4
Set up Procedure: Cable Assembly Attachments 1. LavaBed Humidifier Cartridge: (Figure B) The cartridge must be secured inside the transport incubator enclosure (either attached to the inside wall with heavy duty Velcro or attached to the mattress bracket).
2. Connect grey communication cable to the top left side of the Neo-Pod “T” and connect the Quick Connect Cable to the LavaBed Humidifier Cartridge. Attach the Airway Temperature Sensor to the temperature monitoring port of the circuit on the patient manifold. Note: Airway Temperature Sensor is an optional connection. The Neo-Pod “T” LavaBed Humidifier Cartridge will function normally without the Airway Temperature Sensor installed in the breathing circuit. 3. Connect the color coded terminals of the power cable to a 12 volt DC power source or Plug AC Adaptor into wall outlet. Caution! The Neo-Pod “T” DC power supply cable connection is indicated by the red and black connectors. These must be connected to a 12 volt DC power supply in order to operate properly.
5
Breathing Circuit Connections: 1. Westmed Inc. supplies breathing circuits that are specified for use with the Neo-Pod “T” Transport Humidification System and are intended for use with a variety of ventilators; each circuit package contains a selection of connectors and adaptors to facilitate the connection to the ventilator. 2. Refer to individual circuit product package instructions for use and performance specifications. 3. Ensure the correct circuit assembly is selected for your specific application. 4. Water Feed: Using a syringe, slowly fill the LavaBed Humidifier Cartridge with 20 ml of sterile water. Note: Periodically observe water reservoir and if additional water is required during transport, add as required only with a syringe. To prevent excess water from entering the patient circuit, the cartridge incorporates a safety drain. Warning! Potential unsafe conditions could result if using other than Westmed Inc. specified breathing circuits or accessories with the Neo-Pod “T” Transport Humidification System. Caution! Do not use a water feed set during transport. Motion or conditions that are not level can cause the cartridge’s water level float to malfunction. Caution! If an excessive amount of water is supplied to the cartridge, the safety drain opens and drains the excess water out the bottom of the cartridge. Caution! To avoid excessive condensation do not use the breathing circuit or LavaBed Cartridge Humidifier Cartridge outside the transport incubator. Cautions! 1. Do not operate the Controller if damaged. 2. Do not use in the presence of flammable anesthetic agents or supplemental gases. 3. Do not use a water feed set during transport. 4. Only use 20 ml syringe to fill and refill Lavabed. 5. Neo-Pod “T” Controller requires special precautions regarding Electromagnetic Compatibility (EMC) and must be installed and put into service according to the EMC information provided in this manual. 6
6. Portable and mobile RF communications equipment can affect Neo-Pod “T” Controller. 7. The use of accessories, transducers and cables other than those specified by Westmed, may result in increased EMISSIONS or decreased IMMUNITY of Neo-Pod “T” Controller. 8. The Neo-Pod “T” Controller should not be used adjacent to or stacked with other equipment and if adjacent or stacked use is necessary, the EQUIPMENT should be observed to verify normal operation in the configuration in which it will be used.
Starting and Stopping Treatment: 1. Ensure the Neo-Pod “T” Controller is set up correctly per the individual circuit instructions for use. 2. Fill the LavaBed Humidifier Cartridge with no more than 20 ml of sterile water. 3. Turn the On/Off Knob of the Neo-Pod “T” Controller to “ON”. 4. Set the Temperature Set Knob to the desired temperature 30 ~ 38 °C. Set temperature should be no more than 1°C above incubator temperature. 5. Set ventilator or oxygen supply flow meter to desired settings. 6. Ensure the desired temperature setting in the LavaBed Humidifier Cartridge is obtained before connecting the circuit to the patient’s airway. This will be confirmed when the Target Temp Indicator is on. (Please reference Figure A, page 3). Controls: • On/Off Knob: Turns the power on and off for the Neo-Pod “T” Controller. • Temperature Set Knob: Sets temperature of gas at the LavaBed outlet port. •
•
Airway Temperature Sensor Button: When depressed and held, displays the temperature at the Airway Temperature Sensor. Note: When the Airway Temperature Sensor Button is released the display will resume displaying the LavaBed outlet temperature as explained in Display sections page 8. Silence Alarm Button: Silences the audible alarm for 90 seconds. 7
Alarms: All alarms provide both a visual indication and audible alarm. The audible alarm can be silenced for 90 seconds by pressing the Alarm Silence button; however, the alarm LED will remain illuminated as long as the alarm condition exists. Any alarm will disable the heater in the LavaBed Humidifier Cartridge until the alarm condition is addressed. •
Temperature Sensor Failure Indicator: Indicates that the LavaBed Humidifier Cartridge or Airway Temperature Sensor has failed.
•
40 °C Alarm Indicator: Indicates that either the internal LavaBed Temperature Sensor, or if used, the Airway Temperature Sensor has detected temperatures at or above 40 °C.
Display: (Refer to Figure A on page 3) A)
LavaBed Warming Indicator: This LED is illuminated whenever power is being applied to the heater in the LavaBed Humidifier Cartridge.
B)
Low Temp Indicator: This LED is illuminated when the monitored temperature (LavaBed Humidifier Cartridge or Airway) is 1.5 °C or more below the target temperature on the temperature set.
C)
Target Temp Indicator: This LED is illuminated when the monitored temperature (LavaBed Humidifier Cartridge or Airway) is within 1.5 °C of the target temperature on the temperature set.
D)
High Temp Indicator: This LED is illuminated when the monitored temperature (LavaBed Humidifier Cartridge or Airway) is 1.5°C or more above the target temperature on the temperature set.
8
Cleaning and Maintenance: Periodically the Neo-Pod “T” Controller and Cable may be wiped off using hospital approved germicidal wipes. Warning! Unplug the cable from the Neo-Pod “T” prior to cleaning. Do not put the Controller in water or other liquid as this may cause damage. Servicing: The Neo-Pod “T” should be inspected for proper function by Westmed at a minimum of every five (5) years. While Westmed can provide this service, an inhouse inspection can be performed once per year as follows. 1. Connect the Neo-Pod “T” to the Cable and turn it on. Verify the Temperature Sensor Failure Indicator is illuminated and the audible alarm is turned on. 2. Press the Silence Alarm Button. Verify the audible alarm is turned off. 3. Connect the Cable to the Test Module and verify the Temperature Sensor Failure Indicator is turned off. 4. Set the Temperature Set Knob of the Neo-Pod “T” to 35 ᵒC. Verify that the Target Temp Indicator is illuminated and the LavaBed Warming Indicator is flashing. The Heater LED on the Test Module is flashing simultaneously with the LavaBed Warming Indicator. 5. Put the Airway Temperature Sensor in room temperature. Press and hold the Airway Temperature Sensor Button and verify the Low Temp Indicator is illuminated. 6. Set the Temperature Set Knob of the Neo-Pod “T” to 30 ᵒC. Verify the High Temp Indicator is illuminated, and the LavaBed Warming Indicator and Heater LED on the Test Module are all extinguished. 7. Set the Temperature Set Knob of the Neo-Pod “T” to 38 °C. Verify the Low Temp Indicator; the LavaBed Warming Indicator and the Heater LED on the Test Module are illuminated. 8. Optional: Apply hot air to the Airway Temperature Sensor. Verify 40 ᵒC Alarm Indicator and the audible alarm are turned on. Press the Silence Alarm Button. Verify the 40 ᵒC Alarm Indicator is still Illuminated and the audible alarm is turned off. 9
This device is intended to provide safe and reliable operation. For the best result, it should be operated and maintained in accordance with the instructions provided by Westmed Inc. As with all electrical devices, if any irregularity becomes apparent, you should take caution and have the device inspected by a Westmed Inc. repair specialist. Specifications: • Input Voltage: 12 VDC +2.5 / -1.5
• Compliance: 0.66 mL/kPa full ~ 1.1 mL/kPa empty • Input Current: 1.7 A • Flow Resistance: 3 cm H2O (max.@ 10 L/min, 38 °C.) @ 1 Liter/Second • Output Temperature Range: 30 ~ 38 • Total System Leakage: less than °C @ 100% RH 60 mL/min @ 60 cm H2O • Maximum Output Temperature: 40 °C • Water Reservoir Capacity: 20 ml • Minimum Humidification Output: • Warm-Up Time: 15 minutes max. 33 mg H2O / L/min • Maximum Humidification Output: • Maximum System Operating Pressure: 44 mg H2O / L/min 70 cm H2O • Input Flow Rate: 0~10 L/min @ 50 psi • Operating Temperature Range: 21~38 max °C Warranty: All Westmed products are warranted to be free of defects in material and workmanship for a period of ninety (90) days from date of purchase. Gross Dimensions of Controller: • Weight: 180 grams • Size: 83 mm x 118 mm x 43 mm (Height x Length x Width)
10
Classification according to IEC 60601-1 • Classification of protection: class II • Powered by external 12 volt DC power supply • No internal power supply • No protective earth ground • Type of applied part: BF • Protection from ingress of water: none IPXØ • Mode of operation: capable of continuous operation • No known potential for adverse effects from electromagnetic or other interference between this device and other known devices • No known adverse effects on the performance of the humidification system when exposed to, electrocautery, electrosurgery, defibrillation, X-ray (gamma radiation), infrared radiation, and radiofrequency interference. Guidance and manufacturer’s declaration - electromagnetic emissions The EQUIPMENT is intended for use in the electromagnetic environment specified below. The customer or the user of the EQUIPMENT should assure that it is used in such an environment. Emissions Test Compliance RF emissions Group 1 The EQUIPMENT uses RF energy only for it internal function. CISPR 11 Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. RF emissions Class A CISPR 11 Harmonic Not emissions Applicable IEC 61000-3-2 Voltage Not Fluctuations/ Applicable Flicker emissions The EQUIPMENT is suitable for use in all establishments other than domestic and those directly connected to the public low-voltage power supply network that supplies buildings used for domestic purposes.
11
Guidance and manufacturer’s declaration - electromagnetic emissions The EQUIPMENT is intended for use in the electromagnetic environment specified below. The customer or the user of the EQUIPMENT should assure that it is used in such an environment. Immunity IEC 60601 Compliance Test Test Level Test Electromagnetic environment- guidance Conducted RF 3VRMS 3 VRMS Portable and mobile RF communications equipment IEC 61000-4-6 150 kHz to should be used no closer to any part of the 80 MHz EQUIPMENT, including cables, than the recommended separation distance calculated from the equation applicable to the frequency of the transmitter. Recommended separation distanced: d 3 V/m = 1.2 ÖP at 80 MHz to 800 MHz, d = 2.3 ÖP at 800 Radiated RF 80 MHz to 3 V/m MHz to 2.5 GHz, where P is the maximum output IEC 61000-4-3 2.5 GHz power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m).a Field strengths from fixed RF transmitters, as determined by an electromagnetic site survey, should be less than the compliance level in each frequency b range. Interference may occur in the vicinity of equipment marked with the following symbol:
Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30%. NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people. a Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the EQUIPMENT is used exceeds the applicable RF compliance level above, the EQUIPMENT should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the EQUIPMENT. b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
12
Guidance and manufacturer’s declaration - electromagnetic emissions The EQUIPMENT is intended for use in the electromagnetic environment specified below. The customer or the user of the EQUIPMENT should assure that it is used in such an environment. IEC 60601 Electromagnetic environmentImmunity Test Compliance Test test level guidance Electrostatic ±6 kV contact ±6 kV contact Floors should be wood, concrete or discharge (ESD) ±8 kV air ±8 kV air ceramic tile. If floors are covered with IE 61000-4-2 synthetic material, the relative humidity should be at least 30%. Electrical fast ±2 kV for power ±2 kV for power Main power quality should be that of Transient/burst supply lines supply lines a typical commercial or hospital IEC 61000-4-4 ±1 kV for ±1 kV for environment. input/output lines input/output lines Surge ±1 kV differential ±1 kV differential Main power quality should be that of IEC 61000-4-5 mode mode a typical commercial or hospital ±2 kV common ±2 kV common environment. mode mode Voltage dips, short <5 % UT <5 % UT Main power quality should be that of interruptions and (>95 % dip in UT) (>95 % dip in UT) a typical commercial or hospital voltage variations on for 0,5 cycle for 0,5 cycle 40 % environment. If the user of the power supply input 40 % UT UT EQUIPMENT requires continued lines IEC 61000-4-11 (60 % dip in UT) (60 % dip in UT) operation during power main for 5 cycles for 5 cycles 70 % interruptions, it is recommended that 70 % UT UT the EQUIPMENT be powered from an (30 % dip in UT) (30 % dip in UT) uninterruptible powered supply or a for 25 cycles for 25 cycles <5 % battery. <5 % UT UT (>95 % dip in UT) (>95 % dip in UT) for 5 sec for 5 sec (50/60 Hz) magnetic field IEC61000-4-8
3 A/m
3 A/m
Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. NOTE UT is the AC mains voltage prior to application of the test level.
13
Recommended separation distances between portable and mobile RF communications equipment and the EQUIPMENT The EQUIPMENT is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the EQUIPMENT can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the EQUIPMENT as recommended below, according to the maximum output power of the communications equipment. Rated Separation distance according to frequency of transmitter maximum (meters) output 150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz power d = 1.2 ÖP d = 1.2 ÖP d = 2.3 ÖP of transmitter (Watts) 0.01 0.12 0.12 0.23 0.1 0.38 0.38 0.73 1 1.2 1.2 2.3 10 3.8 3.8 7.3 100 12 12 23 For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer. NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies. NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
14
General Warnings! A warning alerts you to possible injury. Specific Warnings and Cautions appear next to the relevant instructions in the manual. • Use the Neo-Pod “T” only for its intended use as described in this manual. • The Neo-Pod “T” Controller, and LavaBed, should only be used with delivery tubes or accessories recommended by Westmed Inc. Connection of other delivery tubes or accessories could result in patient injury or damage to the device. • Do not submerge the Neo-Pod “T” Controller in water or other liquid as this may cause damage. • Do not use water feed set during transport. • Do not attempt to dismantle the Neo-Pod “T” Controller. There are no userserviceable parts inside. Repairs and internal servicing should only be performed by an authorized service agent. • Do not operate the Neo-Pod “T” Controller if it is not working properly or if any part of the device is damaged. • Keep the cable assembly away from hot surfaces. • Do not pull or allow the Neo-Pod “T” Controller to hang freely from the cable assembly. • Explosion hazard – Do not use in the vicinity of flammable anesthetic vapors or gases. • Follow all precautions when using supplemental oxygen. Disposal: The controller is considered electrical waste and electronic equipment per directive 2002/96/EC and should be recycled as such. For recycling purposes, the controller is RoHS compliant. All disposable products should be treated as bio-hazardous waste and the controller should be treated per the WEEE directive.
15
Manufactured By: 5580 S. Nogales Hwy. Tucson, AZ 85706 USA Telephone: 800-975-7987 Fax: 520-294-6061 www.westmedinc.com PN 76144, Rev. 03 MT Promedt Consulting GmbH Altenhofstr. 80 66386 St. Ingbert, Germany